Energycarrier
H2 The energy carrier
Human beings developed on Earth on the basis of plants, i.e. biomass, as the only energy carrier. The average power consumed by a human body at rest is 0.1 kW and approximately 0.4 kW for a hard working body, delivering about 0.1 kW of work. The consumption of plants by humans and animals did not change the environment because the carbon dioxide, liberated by humans and animals was reabsorbed by the plants in the photosynthesis process. The only mechanical work available from non-living systems were the windmills and the waterwheels, where solar energy was converted into mechanical power.
With the discovery of the steam engine in 1712 by Thomas Newcomen [1] the humanity had for the first time a nonliving machine available, consuming carbon or hydrocarbons and delivering mechanical power on demand. This has lead to the foundation of the industrialized society. The energy for the steam engine was found in the form of mineral coal, solar energy stored in the Earth crust over millions of years.
Coal as a solid energy carrier was later on complemented by liquid crude oil and natural gas. Not only did the state of the energy carrier change, from solid to liquid and finally gaseous, with time but also the amount of hydrogen in the fuel increased from zero to four hydrogen atoms per carbon atom.
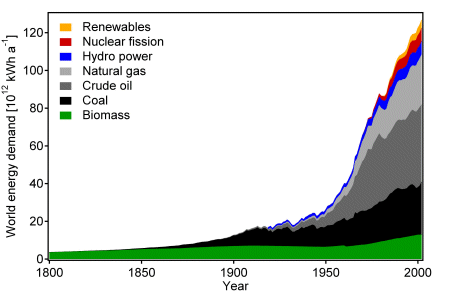
The world energy consumption increased from 5·1012 kWh/year in 1860 to 1.2·1014 kWh/year today. More than 80% are based on fossil fuels, such as coal, oil and gas [2].
Fig. World energy consumption [2]
The population of human beings has increased in the last century by a factor of 4 and the energy demand by a factor of 24. The world wide average continuous power demand is 2 kW/capita. In the USA the power consumption is in average 10 kW/capita and in Europe about 5 kW/capita [3]. Two billion people on Earth do not yet consume any fossil fuels at all. The reserves of fossil fuels on Earth are limited and predictions based on extrapolation of the energy consumption show that the demand will soon exceed the supply. No matter how long the fossil fuels will last their amount is finite. The demand of fossil fuels has also a strong impact on social, political and economic interactions between the various countries. For example two thirds of the crude oil reserves are located in the Near East region, but most of the petrol is consumed in the USA, Europe and Japan. This is the first important fact forcing the world to search for an energy solution not depending on fossile fuels but on unlimited renewable energy.
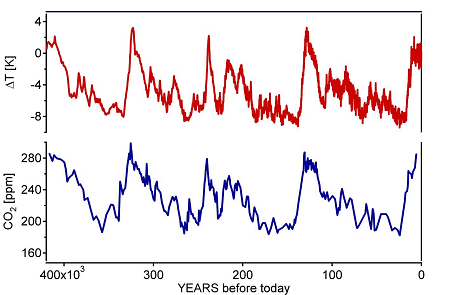
The consumption of fossil fuels together with the deforestation leads to the liberation of 7·1012 kg C/year in the form of CO2. The plants are able to absorb 2·1012 kg/year by means of the photosynthesis process and the same amount is dissolved in the ocean. Therefore the net increase of CO2 in the atmosphere due to human activities is approximately 3·1012 kg/year [4]. This corresponds to an annual increase of 0.4% of the CO2 concentration in the atmosphere. The CO2 concentration in the atmosphere is continuously measured at the Mauna Loa observatory in Hawaii. Due to the seasonal variation of the solar intensity the CO2 concentration shows oscillations. The plants absorb more CO2 in the growth period, the summer time than during the winter. However, plants are not able to absorb all the additionally liberated CO2 in real time. Carbon dioxide is a greenhouse gas and causes an increase of the average temperature on Earth. A careful investigation of the climate and atmospheric history of the past 420’000 years from the Vostok ice core in the Antarctica has shown that the variations of the temperature on Earth correlates with the variations of the concentration of greenhouse gases in the atmosphere. This correlation, together with the uniquely elevated concentrations of CO2 today, is of great relevance with respect to the continuing debate on the future of Earth’s climate.
Fig. The Vostock sensation, average temperature on Earth and CO2 concentration in the atmosphere over the last 400000 years.[5]
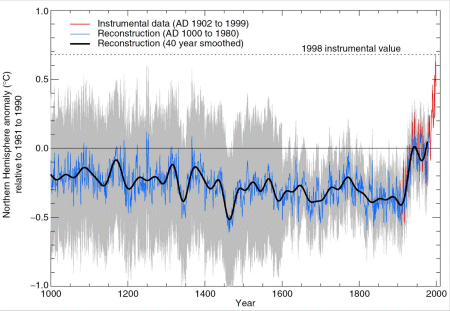
Currently we are in a period of decreasing solar activity and therefore the average surface temperature on Earth should decrease. However, since 100 years the temperature on Earth increases. Furthermore, since 10 years an unusual steep increase of the average temperature versus time is observed. This is the second important reason for the necessity to develop a new energy carrier, which is free of carbon.
Fig. Development of the average temperature on Earth over the last 1000 years.
Fossil energy has a very strong influence on the development of a society and the standard of living. A dependency of the gross national product on the average amount of energy consumed per person can be found. Countries with a low energy consumption per person have a low gross national product and vice versa. This lead to the conclusion, that today the economic gain of the industrialized world is to a significant part due to the energy from fossil fuels.
Fossil fuels are energy carriers given to the human beings by nature. Basically we know two types of energy carriers. The first type is a reversible system which can be charged with energy in the form of mechanical or electrical work and delivers the energy again on demand. Examples for such a system are capacitors, batteries and flywheels. We can define a charge and discharge efficiency and the overall efficiency of the system is the product of all partial efficiencies. The energy density in such systems is given by the physical properties of the active material and is in general limited to 1 to 2 eV per atom. The second system stores energy by means of the reduction of a compound liberating oxygen to the atmosphere and producing a semi stable product e.g. photosynthesis (6CO2 + 6H2O ® C6H12O6 + 6O2) or 2ZnO ® Zn + O2. The product can be reacted with oxygen again liberating energy and leading to volatile or non-volatile compounds. In case the compound is volatile a natural process in the atmosphere should transport it. The advantage of the second system over the first one is the natural transport and the storage of the oxygen and the volatile products in the atmosphere. Therefore, rather high-energy storage densities are possible and can exceed in many cases the storage densities of the first system. But more processes are involved and this limits the overall energy efficiency of the system.
The natural cycles in the atmosphere are able to transport only a few compounds namely oxygen, nitrogen, water and carbon dioxide. Many more gases could of course be transported, however, they would significantly affect life on Earth.
Considering the historical development of energy carriers towards more hydrogen rich fuels and the necessity to avoid the carbon dioxide emission that causes the greenhouse effect, one concludes hydrogen should be the energy carrier of the future:
C (coal) ® -CH2– (oil) ® CH4 (natural gas) ® H2 (hydrogen)
This series also shows a development going from a solid to a liquid and then finally to a gas state energy carrier. Hydrogen occurs on Earth chemically bound as H2O in water and some is bound to liquid or gaseous hydrocarbons. The production of hydrogen by means of electrolysis consumes electricity, which is physical work. Therefore, a primary energy source is necessary to produce hydrogen. In contrast to the biomass produced by all plants using sunlight, there are only a few natural processes leading directly to hydrogen gas. The fundamental question to be answered is what are the possible sources of energy for a hydrogen-based society.
The answer today is nuclear fission or nuclear fusion. The human controlled nuclear fission is limited by the mining of the materials used in nuclear reactors and the handling of the products of the fission reaction. Furthermore, the reserves of highly concentrated fission materials in the Earth crust are finite and would not allow to produce the world energy demand for more than a century. Natural fission in the Earth leads to a reasonable amount of heat. This heat is the source of geothermal power used in suitable regions, like Iceland, as primary energy source. However, estimates of the potential amount of energy from geothermal sources show that they cover only a few percent of the actual world wide energy demand.
On a geological time scale the only source of primary energy is the nuclear fusion of hydrogen. The two options are terrestric or extraterrestric fusion on the sun. The terrestric fusion is human controlled and could deliver the energy in a centralized and concentrated form on a high temperature. However, today the necessary condition, the Lawson product, i.e. the product of plasma density, confinement time and temperature larger than 6·1028 K·s·m-3, can only be achieved for a very limited time. The Sun on the other hand delivers the fusion energy over the hole surface of the Earth in a continuous oscillating way with a frequency of 24 hours on a base frequency of one year. The energy arrives on Earth with a rather low average intensity of 165 W m-2. The challenge is to convert and concentrate the energy by means of hydropower, windpower, solar-thermal, photovoltaics or even biomass. The conversion via photovoltaics for example can be estimated as follows: The solar constant is 1.369 kW·m-2 and approximately 50% of the solar radiation reach the surface of the Earth. Photovoltaic systems have an efficiency of approximately 10%. In the best case half of the time is night and therefore, under ideal conditions about 473’000 km2 (80 m2/capita) covered with photovoltaic cells are necessary to produce the world energy demand of today. This is a square with a side length of 700 km located in the northern Africa for example.
Fig. Surface area to be covered in the Sahara by photovoltaic cells in order to provide the world energy demand in the year 2020 (red square).
Energy from sunlight is converted into electricity for example by means of photovoltaic cells. Electricity from a renewable energy source is used for the electrolysis of water. Electrolysis at ambient temperature and pressure requires a minimum voltage of 1.481V and therefore, a minimum energy of 39.7 kWh·kg-1 hydrogen. Today electrolyser systems consume approximately 47 kWh·kg-1 hydrogen, i.e. the efficiency is approximately 82%. The oxygen is released in the atmosphere and hydrogen is stored, transported and distributed. Finally, hydrogen together with the oxygen is combusted and the energy is released as heat and work leaving water or steam into the atmosphere. Therewith the hydrogen cycle is closed.
Electrolysis of water is an established technology and is used today to produce high purity hydrogen. The only impurities in the hydrogen gas from electrolyses are water and oxygen. The chemical energy per mass of hydrogen (39.4 kWh·kg-1) is three times larger than that of other chemical fuels, e.g. liquid hydrocarbons (13.1 kWh·kg-1). In other words, the energy content of 0.33 kg of hydrogen corresponds to the energy content of 1 kg of oil. There is, however, a technical and an economic challenge to overcome before the hydrogen energy economy becomes reality.
The technical challenge is the real time production, the safe and convenient storage and the efficient combustion of hydrogen. In order to satisfy the worlds demand of fossil fuels, more than 3·1012 kg hydrogen would have to be produced per year. This is roughly 100 times the current hydrogen production.
The economic challenge is the cost of the hydrogen production. World economy today is based on free energy naturally stored over millions of years. The price we are used to pay for fossil fuels is the mining cost only. In order to adapt the world to a synthetic fuel like hydrogen the world economy has to be convinced to pay for the conversion of solar energy into a fuel, too.
References
[1] Encyclopaedia Britannica, Incorporated; 15th ed edition (August 15, 2001); S. S. Wilson, Spektrum der Wissenschaft (Okt. 1981), pp. 99-109.
[2] Jean-Marie Martin-Amouroux, IEPE, Grenoble, France, private communication (2003); BP Statistical Review of World Energy 2004.
[3] International Energy Agency (IEA, AIE), “Key World Energy Statistics” 2002 Edition.
[4] Climate Change 2001: Synthesis Report, Robert T. Watson (ed.), Published for the Inter-governmental Panel on Climate Change IPCC, (2001), Cambridge University Press, Cambridge, UK
[5] Petit J R; Jouzel J; Raynaud D; Barkov N I; Barnola J M; Basile I; Bender M; Chappellaz J; Davisk M; Delaygue G; Delmotte M; Kotlyakov V M; Legrand M; Lipenkov V Y; Lorius C; Pepin L; Ritz C; Saltzmank E; Stievenard M, “Climate and atmospheric history of the past 420,000 years from the Vostok ice core, Antarctica”, NATURE, VOL 399 (1999), pp. 429-436